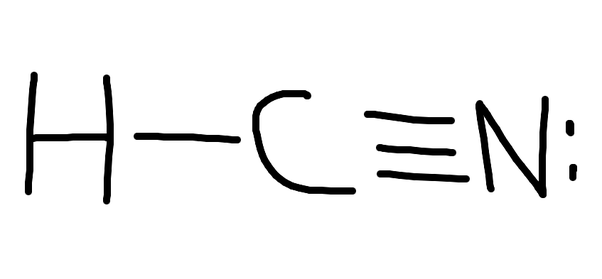
Lewis Diagram For Hcn
Drawing Lewis diagrams. A Lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. When constructing a Lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms.
[DIAGRAM] Lewis Dot Diagram For Hydrogen Cyanide
To determine the HCN Lewis Dot Structure first we need to look for valence electrons in individual atoms. Hydrogen (Atomic number = 1 and electronic configuration = 1) belongs to the 1 st group of the periodic table and consists of only 1 electron.

HCN Lewis Structure (Hydrogen Cyanide) Molecules, Chemical formula, Lewis
HCN, hydrogen cyanide, is a volatile and poisnous compound with distinguished bitter odor. It is linear molecule with a triple bond between C and N atom and has bond angle of 180 degrees.. The Valence Bond thoery simply explains the bond formation just like lewis dot structure, but instead it explains the bonding in terms of covalent bond by.
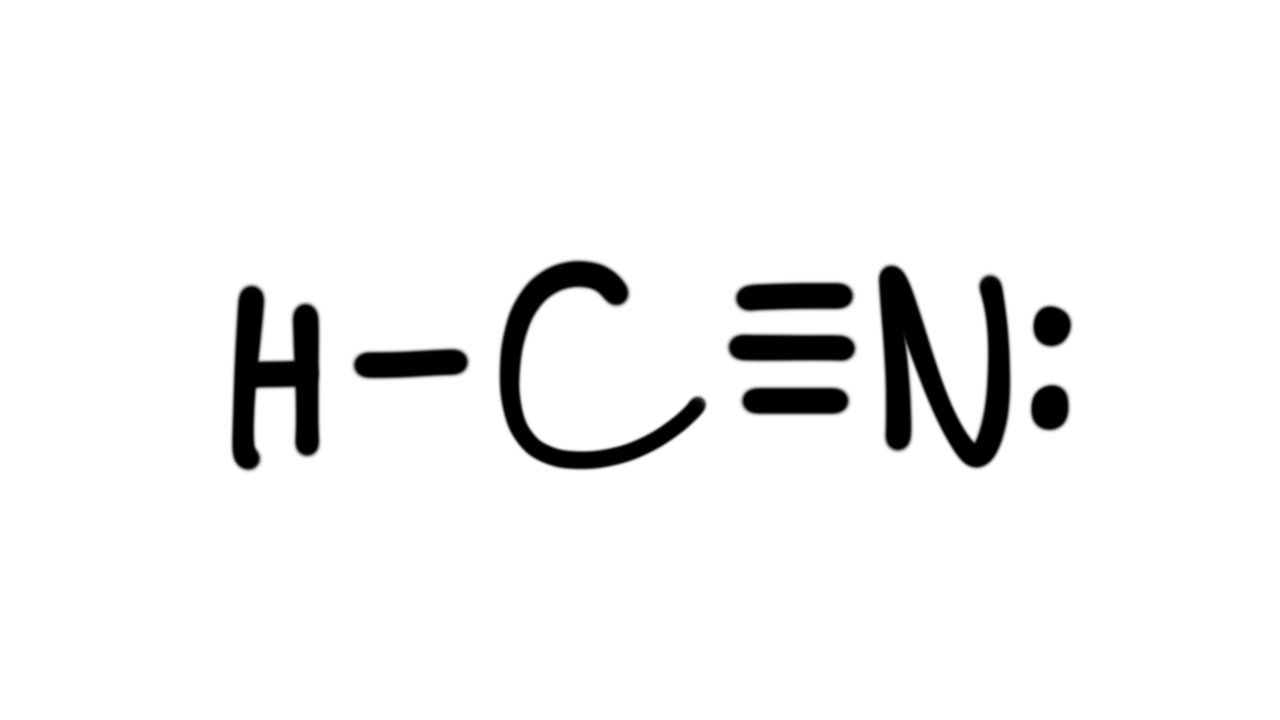
Lewis Diagram For Hcn
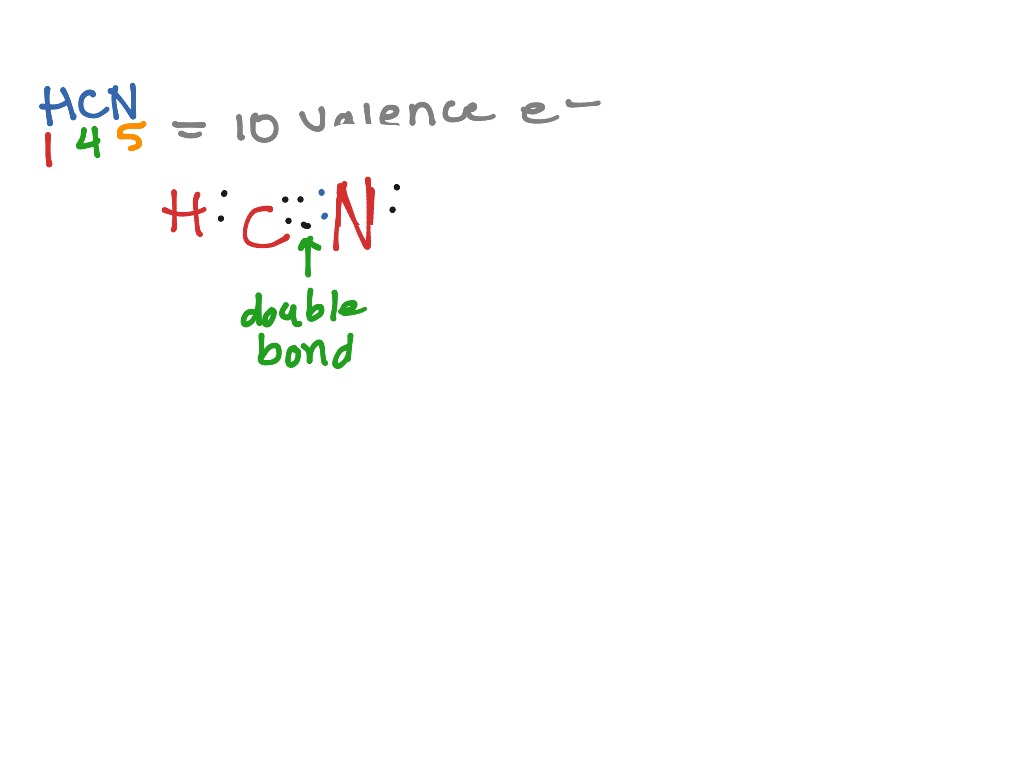
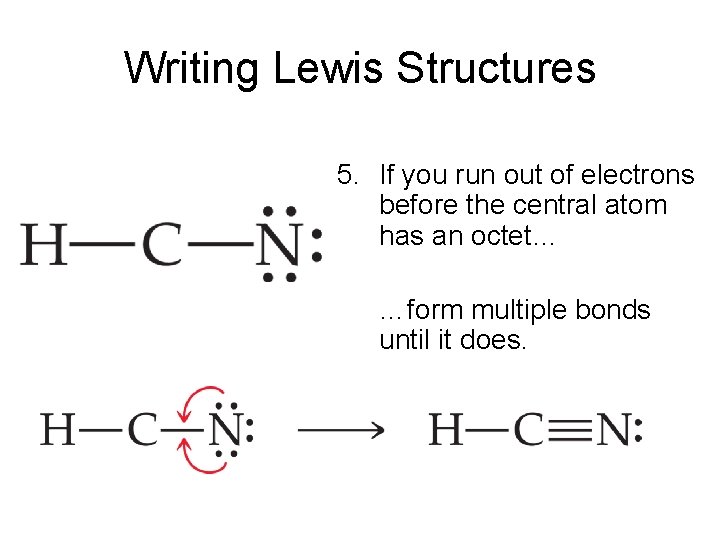
Transcript: For the HCN Lewis structure we have one valence electron for Hydrogen, we have four for Carbon, and we have five for Nitrogen, for a total of ten valence electrons for the HCN Lewis structure. We'll put the Carbon in the center, because it's less electronegative than the Nitrogen, and Hydrogens always go on the outside of Lewis structures.
Lewis Diagram For Hcn
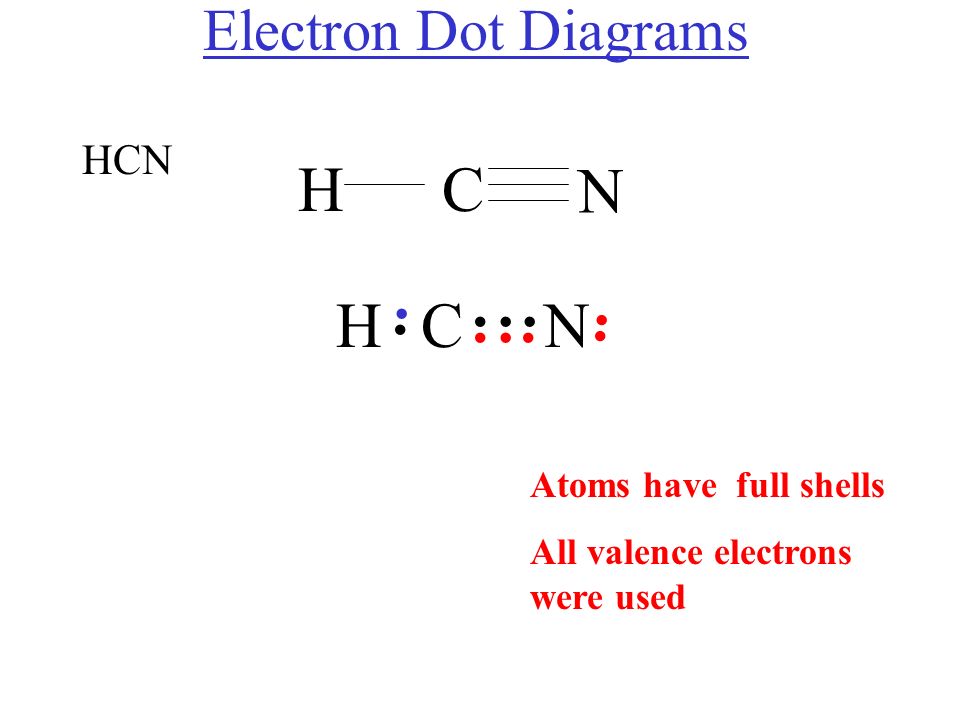
A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.

Hcn hydrogen cyanide molecule Royalty Free Vector Image
Use these steps to correctly draw the HCN Lewis structure: #1 First draw a rough sketch #2 Mark lone pairs on the atoms #3 Calculate and mark formal charges on the atoms, if required #4 Convert lone pairs of the atoms, and minimize formal charges #5 Repeat step 4 if needed, until all charges are minimized, to get a stable Lewis structure

HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and Polarity Techiescientist
The HCN Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the HCN molecule. The geometry of the HCN molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory (VSEPR Theory), which states that molecules will choose the HCN geometrical shape in which the electrons have from one another.
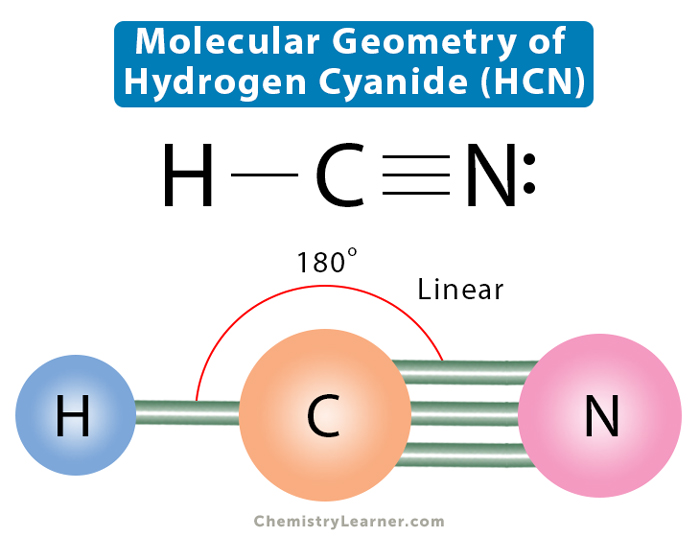
Molecular Geometry, Lewis Structure, and Bond Angle of HCN
Hydrogen Cyanide is a colorless, flammable, and poisonous chemical liquid. Represented by the chemical formula, HCN is one of those molecules that has an interesting Lewis structure. This liquid is used in electroplating, mining, and as a precursor for several compounds. HCN Lewis Structure (Hydrogen Cyanide) Watch on 0:00 / 2:55
Hcn Lewis Structure Bonds Draw Easy
Solution. Steps for Writing Lewis Structures. Example 3.4.1 3.4. 1. 1. Determine the total number of valence electrons in the molecule or ion. Each H atom (group 1) has 1 valence electron, and the O atom (group 16) has 6 valence electrons, for a total of 8 valence electrons. 2.

Lewis Dot Diagram Of Hcn
A quick explanation of the molecular geometry of HCN including a description of the HCN bond angles.Looking at the HCN Lewis structure we can see that there.
Hcn Lewis Structure Bonds Draw Easy
The Lewis Structure (Lewis Dot Diagram) for HCN. 1. Count electrons 2. Put least electronegative atom in centre.more.more Lewis Diagrams Made Easy: How to Draw Lewis Dot.
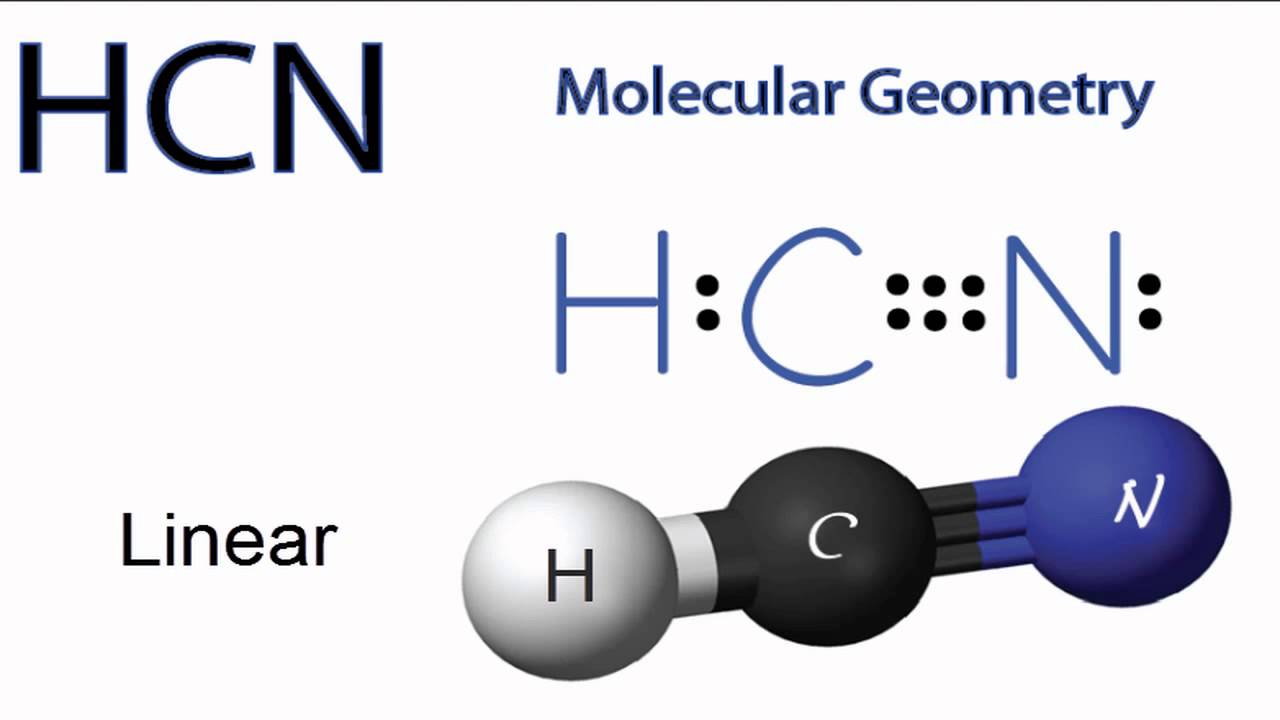
HCN Molecular Geometry YouTube
How to Draw Lewis Structures: Five Easy Steps Wayne Breslyn 2.7M views 10 years ago CO2 Lewis Structure - How to Draw the Dot Structure for Carbon Dioxide

Hcn Lewis Structure Bonds Draw Easy
What is this molecule and what is it used for? HCN, hydrogen cyanide, is rather poisonous. HCN is a gas used primarily in chemical synthesis, mining, and polymer manufacturing. But serious, it's dangerous, so stay away unless you are a legit chemist. Method 1: Step method to draw the Lewis structure of HCN.
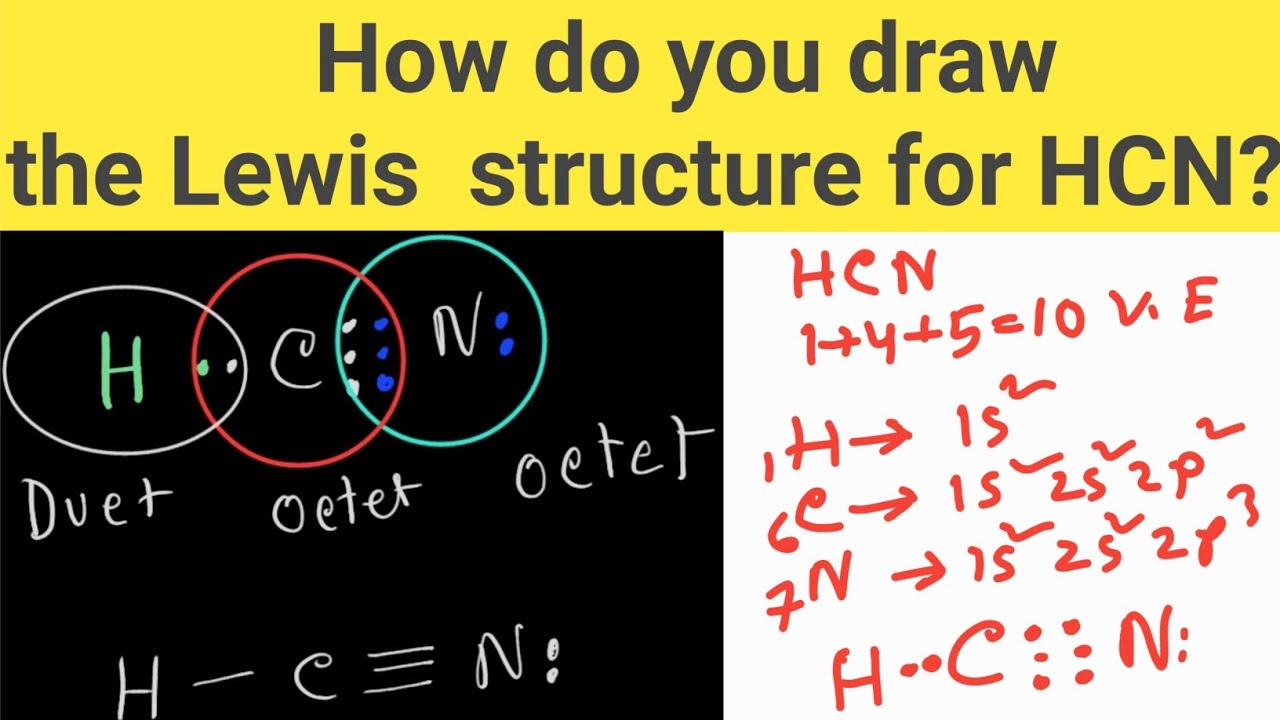
How do you draw the Lewis structure of HCN (hydrogen cyanide)? HCN Lewis Dot Structure YouTube
Molecular Structure of Compounds 6.2 Lewis Structures We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table.

HCN Lewis Structure How to Draw the Dot Structure II lSCIENCE ll NCERT ll Rohit Sir YouTube
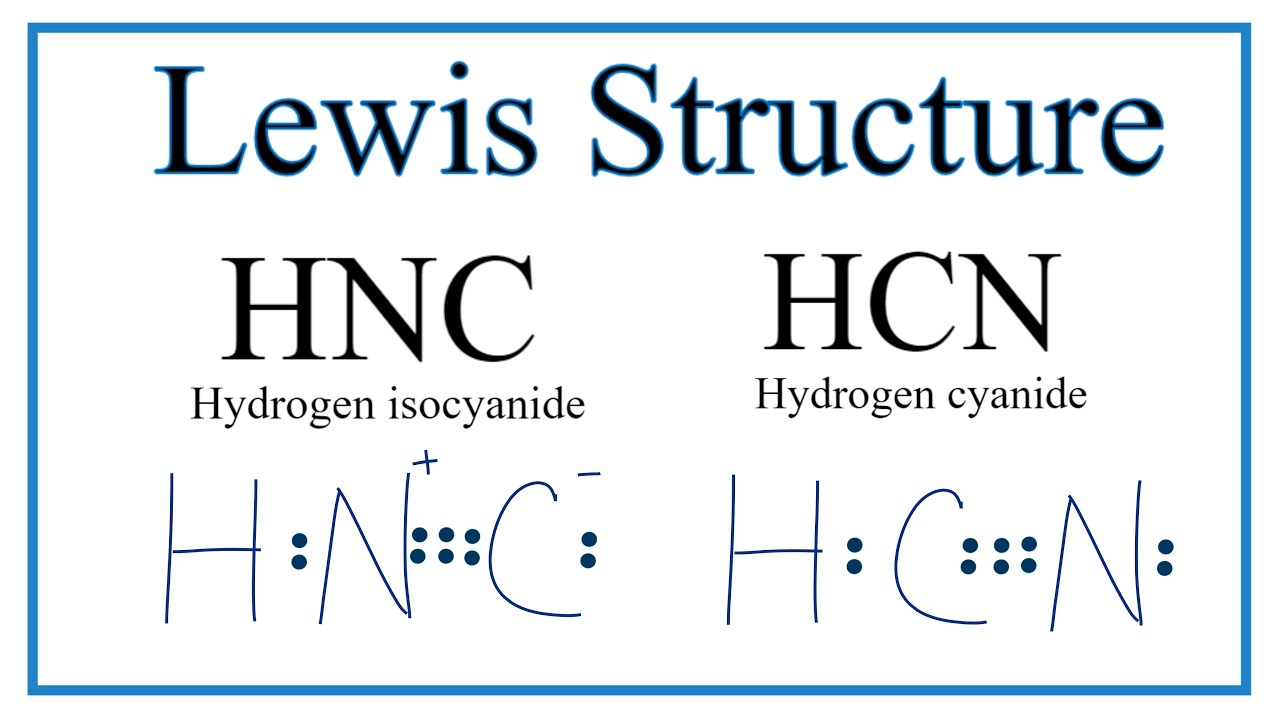
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule.. Example: calculating the formal charges in HCN and HNC. For the arrangement HCN, the Lewis structure: H-C\(\equiv\)N:

4. 11C04.1 PSV 1 Lewis structure of HCN YouTube
See the diagram below: Now you can see that the central atom here is Carbon because it is easy for Carbon to become stable as it is the least electronegative of all. However, hydrogen is the least electronegative but it cant be a central atom because it has only one spare electron.